PHARMACEUTICAL COLLABORATORS

About Mary Crowley Cancer Research
 Mary Crowley Cancer Research (MCCR) is an independent, nonprofit oncology research center established in 1997. Founded by Mary C. Crowley, our mission is to bring hope to cancer patients through innovative clinical trials while advancing treatment for patients in the future.
Mary Crowley Cancer Research (MCCR) is an independent, nonprofit oncology research center established in 1997. Founded by Mary C. Crowley, our mission is to bring hope to cancer patients through innovative clinical trials while advancing treatment for patients in the future.
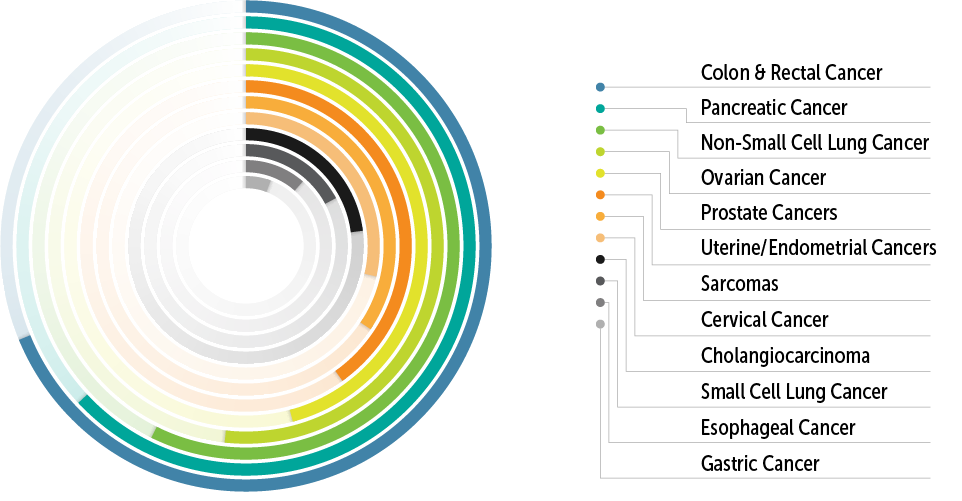
MCCR has conducted 740+ clinical trials for 7400+ patients in collaboration with 291+ different pharmaceutical partners. MCCR is designed, equipped, and staffed for conducting early phase solid tumor and hematology trials, including performing 129+ first-in-human trials. Outpatient and inpatient hospital support, and a dedicated referral and consult team. MCCR has completed phase I and phase II, industry funded studies including first-in-human, drug-drug interaction, food effect, bioequivalence, and intensive EKG monitoring studies. This site has experience using gene therapies, cytokines, cytotoxic agents, cellular therapies, small molecules, viral therapies, monoclonal antibodies, antibody drug conjugates, immune therapy, and vaccines.
An Accelerated Approach to Cancer Research
| Study Start-Up | Patient Population | Data |
|
|
|

|
The Clinic
|
The Administrative Offices
|
Processes
Click below to access an overview of our site’s processes (pdf)
FOR MORE INFORMATION, CONTACT:
Alisha Doege, Study Development Manager, and Tina Nghiem, Associate Director, Clinical Trial Development, at pharma@marycrowley.org





